CardioFocus Announces Publication of 1-Year ECLIPSE AF Clinical Trial Results
1-year results demonstrated 90.2% freedom from atrial arrhythmia in paroxysmal AF patients using CardioFocus’ Centauri System with a proprietary monopolar PFA waveform
MARLBOROUGH, Mass. --(BUSINESS WIRE)
CardioFocus, Inc., a medical device company dedicated to advancing ablation treatment for cardiac arrhythmias, today announced the publication of the 12-month results from the ECLIPSE AF trial in Circulation: Arrhythmia & Electrophysiology, demonstrating an overall 90.2% 12-month freedom from clinically significant atrial arrhythmia among paroxysmal atrial fibrillation (AF) patients undergoing de novo pulmonary vein isolation (PVI) using three commercial contact-force sensing focal catheters with the Centauri™ System’s optimized WAVE1™ pulsed field ablation (PFA) waveform. The system, which has since received CE mark and is commercially available within the EU and UK where over 6000 patients have been successfully treated, was used with the Abbott TactiCath™ Sensor Enabled, Boston Scientific INTELLANAV STABLEPOINT™, and Johnson & Johnson THERMOCOOL SMARTTOUCH™ ablation catheters. Paroxysmal and persistent AF patients were treated in the study and demonstrated an overall 80.2% 12-month freedom from clinically significant atrial arrhythmia. The waveform was optimized with invasive 90-day remapping and demonstrated an overall chronic PVI durability rate of 89%, per pulmonary vein, in patients treated within the optimized PFA cohorts.
This press release features multimedia. View the full release here: https://www.businesswire.com/news/home/20250114082410/en/
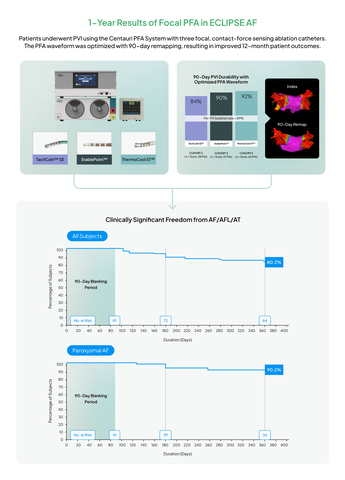
Graphical Abstract for 1-Year ECLIPSE AF Results (Graphic: Business Wire)
“Waveform science is crucial to deliver safe and effective therapy with pulsed electric fields,” said Dr. Ante Anic, Head of Electrophysiology, University Hospital Split, Split, Croatia, and Principal Investigator of the study. “Along with my extensive clinical experience with PFA technologies, the study results in this peer-reviewed publication confirm that using a traditional point-by-point workflow with focal catheters and an optimized waveform will deliver excellent clinical results. In only a few seconds per application we can achieve excellent ablation depths that produce durable PVI lesions and high rates of freedom from AF while retaining an excellent safety profile.”
“Performing remapping in this study was critical to optimizing the PFA waveform and procedural workflow,” said Dr. Johan Vijgen, Division Chief of Electrophysiology at Jessa Hospital, Hasselt, Belgium, and Co-Investigator in the study. “The iterative process of assessing and improving the index ablation treatment strategy resulted in durable PVI and exceptional long-term arrhythmia survival rates.”
CardioFocus has leveraged the scientific foundation and successful patient outcomes of the ECLIPSE AF study in the ongoing trials for the large-area focal QuickShot™ Nav Catheter, and the OptiShot™ PFA Balloon Catheter – a next-generation ultra-compliant PFA balloon for PVI. “We are committed to advancing the science of PFA in electrophysiology. We customize our proprietary pulsed electric field waveforms with each catheter system to deliver precise treatments to improve patient outcomes and we are thrilled with the excellent results in the ECLIPSE AF clinical study,” said Steve Ogilvie, Chief Executive Officer at CardioFocus.
ECLIPSE AF (NCT# 04523545) was a multi-center, single-arm, prospective first-in-human study designed to evaluate the safety and performance of the Centauri System with commercial focal ablation catheters. A total of 82 patients were enrolled and treated in the study at 2 centers in the European Union.
Link to the study: https://www.ahajournals.org/doi/10.1161/CIRCEP.124.012794
About CardioFocus, Inc.
Headquartered in Marlborough, MA, CardioFocus is a medical device innovator and manufacturer dedicated to advancing ablation treatment for cardiac disorders such as atrial fibrillation, the most common heart arrhythmia. For more information, visit CardioFocus.com.
View source version on businesswire.com: https://www.businesswire.com/news/home/20250114082410/en/
Media Relations
Pete Bell
[email protected]
Copyright Business Wire 2025
Information contained on this page is provided by an independent third-party content provider. XPRMedia and this Site make no warranties or representations in connection therewith. If you are affiliated with this page and would like it removed please contact [email protected]

