VisiRose Announces $3 Million Seed Financing Round to Advance Ocular Therapeutics
KNOXVILLE, TN / ACCESSWIRE / January 14, 2025 / VisiRose Inc. ("VisiRose" or the "Company"), a privately-held, clinical-stage biotechnology company developing novel ocular therapeutics, today announced a $3 million seed financing round for VisiRose. The funds from this round will be directed toward advancing the Company's lead investigational program, particularly facilitating critical regulatory milestones of the clinical development of its therapies for corneal diseases and other severe ocular conditions.
The proceeds from this financing round will support several key initiatives, including:
Completing a pre-investigational new drug (IND) submission meeting with the U.S. Food and Drug Administration (FDA) for VisiRose's Rose Bengal Photodynamic Antimicrobial Therapy (RB PDAT), a groundbreaking, non-invasive, investigational treatment for infectious keratitis and other serious eye infections.
Submitting an IND application for investigational drug PV-305, targeting corneal blindness (RB) through photodynamic therapy (PDAT), and working toward its acceptance by the FDA.
Manufacturing an initial clinical supply of PV-305, enabling the Company to initiate clinical trials, undertake expanded access, and move closer to regulatory approval.
VisiRose is leveraging the novel ocular RB PDAT research from the University of Miami's Bascom Palmer Eye Institute and Ophthalmic Biophysics Center, in collaboration with Provectus Biopharmaceuticals (OTCQB: PVCT). At the heart of the Company's therapeutic platform is Provectus's bioactive synthetic small molecule, Rose Bengal Sodium (RBS), to treat a range of sight-threatening ocular conditions with high unmet medical needs.
"We are pleased to complete this financing round to support the continued progress of our ophthalmology drug pipeline," said Dominic Rodrigues, Acting Chief Executive Officer of VisiRose. "The funding will enable us to advance our lead drug product candidate PV-305 through critical regulatory steps, ultimately moving us closer to providing patients with innovative therapies for corneal diseases and other serious ocular conditions."
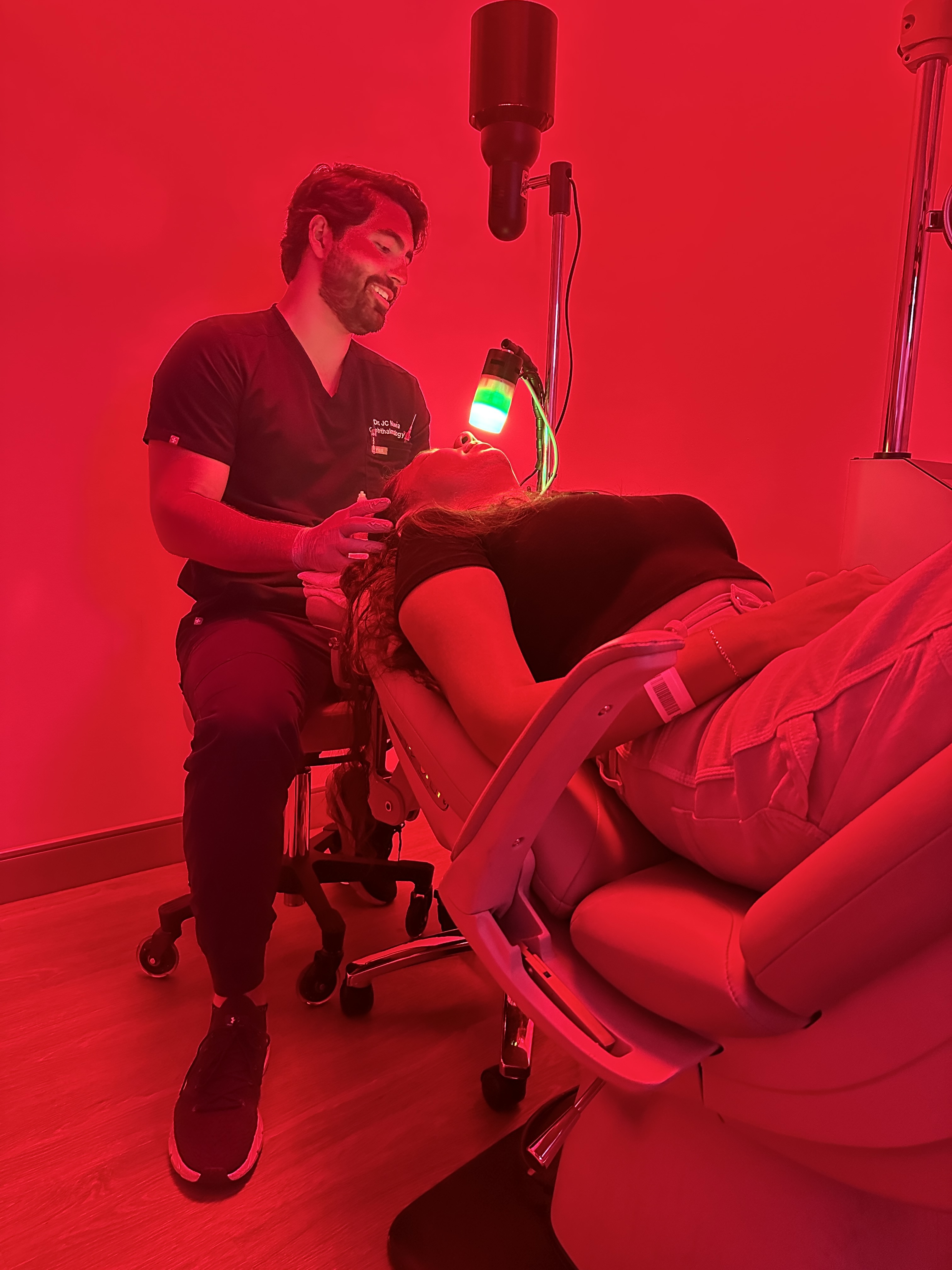
Courtesy of Bascom Palmer Eye Institute
About VisiRose
VisiRose is a newly launched, clinical-stage biotechnology company of the University of Miami and Provectus Biopharmaceuticals, focused on commercializing the Miller School of Medicine's Bascom Palmer Eye Institute and its Ophthalmic Biophysics Center's innovative ocular research using Provectus's bioactive synthetic small molecule Rose Bengal Sodium (RBS). For more information, please visit: https://visirose.com.
Contact:
VisiRose, Inc.
Dominic Rodrigues
Acting Chief Executive Officer
[email protected]
For Media Inquiries:
Ira M. Gostin
[email protected]
775-391-0213
SOURCE: VisiRose, Inc.
Information contained on this page is provided by an independent third-party content provider. XPRMedia and this Site make no warranties or representations in connection therewith. If you are affiliated with this page and would like it removed please contact [email protected]

